Which of the Following Bonds Is Least Polar
If one atom is significantly more electronegative than the other. N is about 30.

Which Bond Is More Polar Youtube
The polarity of a bond depends on electronegativity difference between the two atoms sharing the bonding electrons.

. Mg-O C-O O-O Si-O N-O. Dipole moments result from the unequal distribution of. Correct option is B C H bond is least polar as the.
A covalent bond in which the bonding electrons are equally attracted to both bonded atoms. Which of the following statements is incorrect. The least polar bond is CC the reason is equal electronegativity the ability of an element to attract electron.
H S has least polarity in the bond because the electronegativity difference between H and S is the least. Which of the following statements is incorrect. Ionic bonding results from the transfer of electrons from one atom to another.
The electronegativity difference in oxygen and flourine is 05 and it is the least polar when you compare to all others. Two very e- atoms are covalently bound c. H-N-C ö-H H H 180 120 1095 90.
The smallest difference will be the most covalent bonds least polar. Which of the following statements best describes a relatively polar bond. Select the answer choice that correct identifies if the bonds formed would be.
Which of the following bonds is the least polar. Which of the following molecules has a dipole moment. Increasing the electronegativity difference lead.
It also explains how to rank the bonds from least polar to most po. The least polar bond is the one with the least difference in electronegativity between the two elements. See full answer below.
EqH - 21 N - 30 O - 35 B - 15 Cl - 30 F -. Which of the following bonds is least polar. Given the following bond.
Which of the following bonds is least polar. H - O. More the polar bond more is the electronegativity difference and converse is.
Na is about 09 and Cl is about 35 and the difference is about 26. Which of the following will be least polar. Which is the least polar bond.
Na - O. A very e- atom and a weakly. Which of the following bonds would be the least polar yet still be considered polar covalent.
Non polar covalent bond. We begin by using the electronegativity values to determine the least polar bonds. C - Cl.
The polarity of a bond describes how evenly or unevenly the bonding electrons are shared between two atoms. B Dipole moments result from the unequal distribution of. C-CI C-O C-F C-H What are the bond angles around the circled carbon in the compound shown here.
The lower the difference in electronegativity between the bonded atoms the less polar the bond. A χ 098 - 258 160. This organic chemistry video tutorial explains how to determine which bond is more polar.
Atoms ability to attract and hold electrons. H 2 O. C-Se CO Cl-Br OO N-H C-H.
Two weakly e- atoms undergo ionic bonding b. B - F. A Ionic bonding results from the transfer of electrons from one atom to another.
A CO b HC c PCl d NaCl e They are all nonpolar.
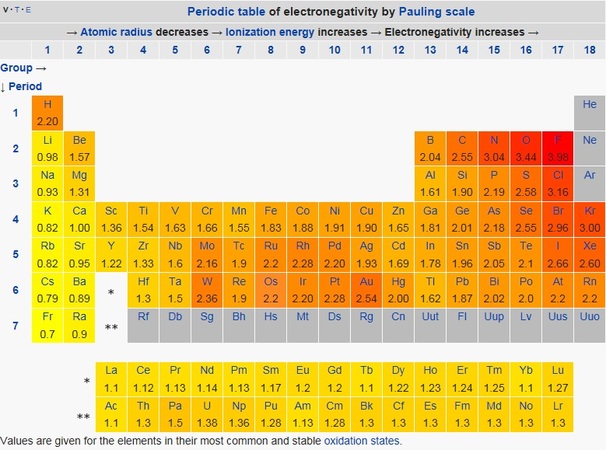
Science Skool Electronegativity And Polarity

Chapter 8 Ionic And Covalent Bonding Ppt Video Online Download
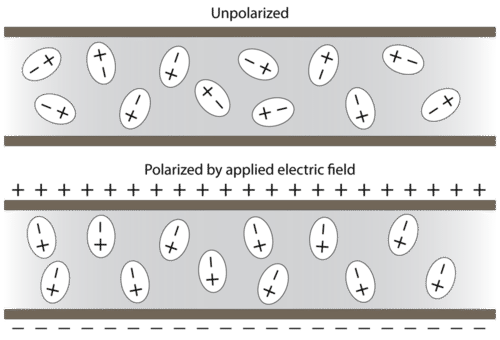
4 9 Polar Molecules Chemistry Libretexts
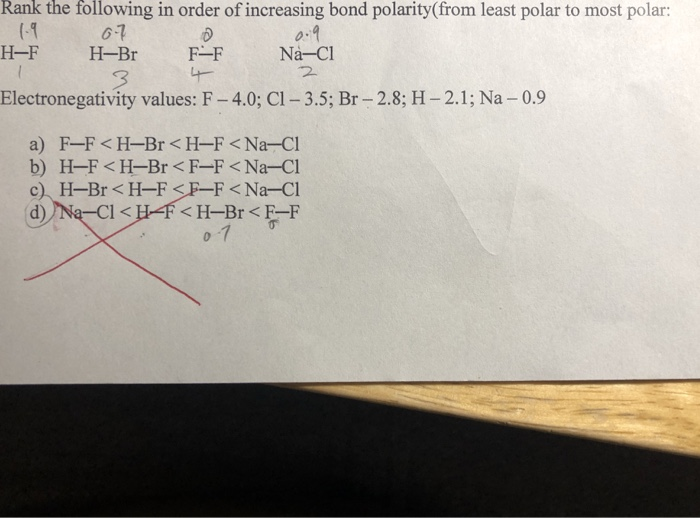
Solved Rank The Following In Order Of Increasing Bond Chegg Com
Comments
Post a Comment